This website is for healthcare professionals and other relevant decision makers only.
ClearRead CT and Calantic™ work in tandem to enhance radiology practices by providing advanced tools for accurate and efficient analysis of chest CT scans.
ClearRead CT empowers radiologists to analyze actionable data with improved accuracy and quality, utilizing patented vessel suppression technology to deliver an unobstructed view of the chest. This capability is essential for the early detection and characterization of five types of nodules, significantly enhancing the diagnostic process.
When integrated with Calantic™, which offers a unified platform for image visualization and seamless workflow, healthcare centers can optimize their operations and improve patient outcomes through advanced imaging solutions. Additionally, both ClearRead CT and Calantic™ comply with legal requirements for the upcoming lung cancer screening program in Germany, ensuring adherence to the following regulatory requirements and experts' opinions:
-

Comprehensive nodule detection capabilities (solid, subsolid, or ground glass)
-

Volume doubling time
-

Provides structured reports
-

Calcium Scoring
-

Lung RADS 2022*
-

Lung Texture Analysis*
*pending CE mark
20
Years of experience
4M
Exams annually worldwide
550
Customers globally
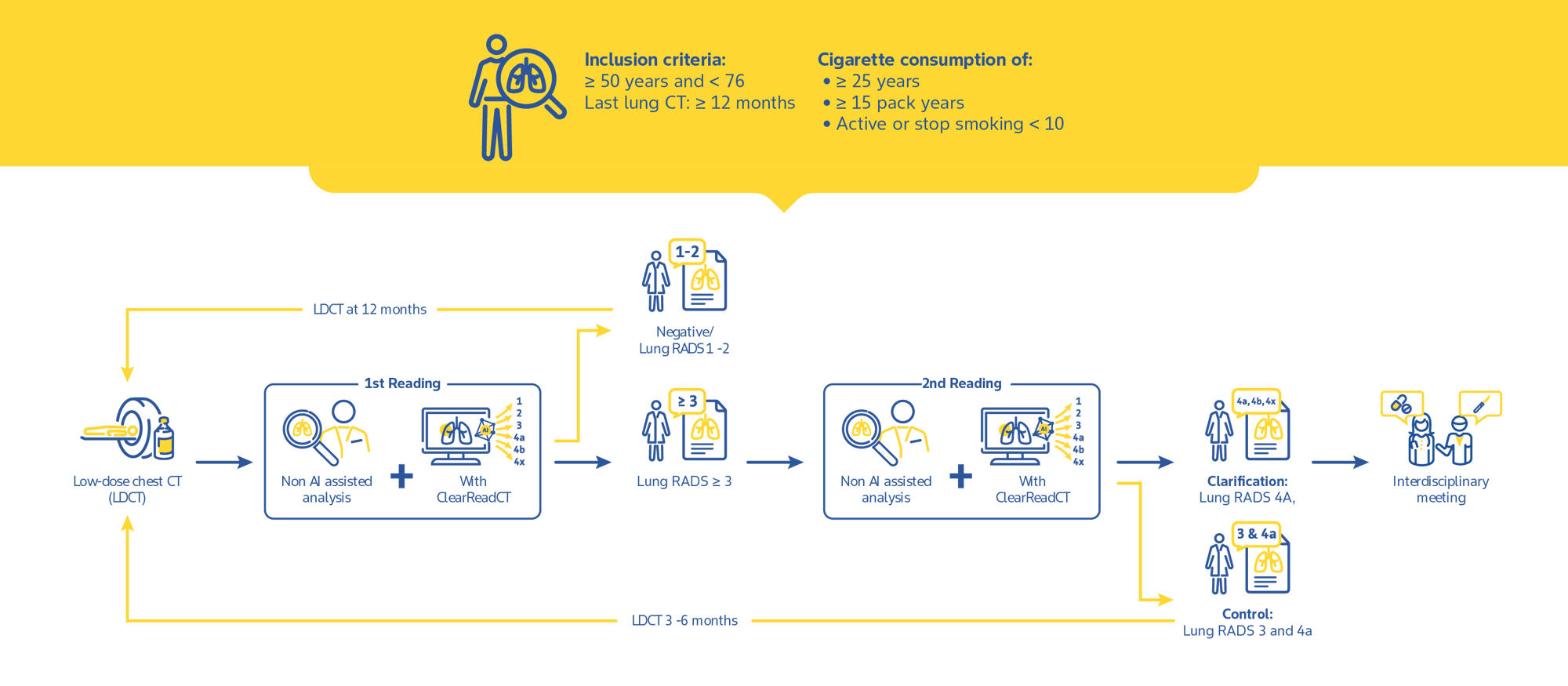
Vessel suppression increases nodule detection rate, improves interreader agreement, and reduces reading time in chest CT of oncologic patients.1
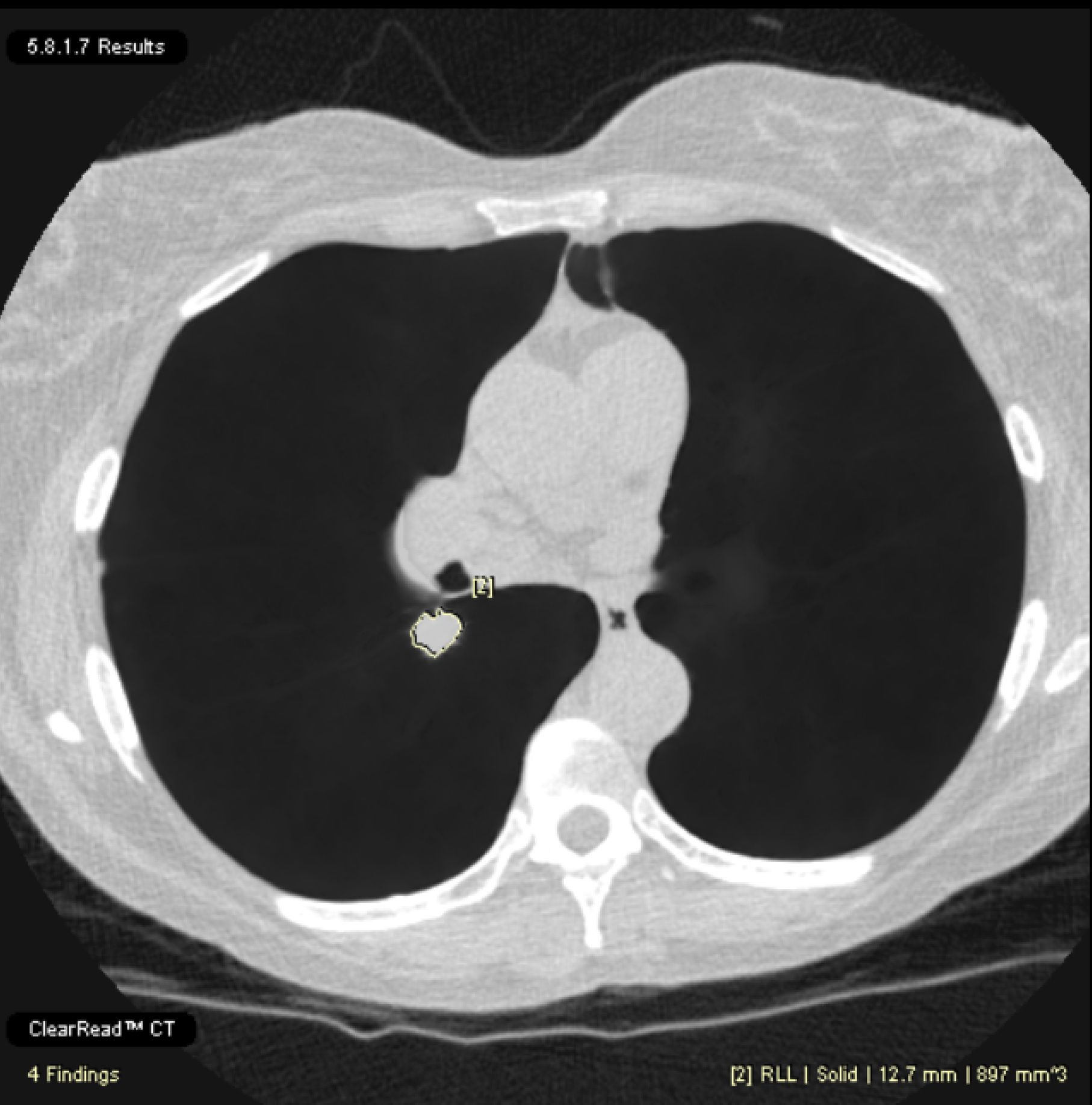
Provides measurements for each detected region of interest including location, type, volume, maximum, minimum, and average axial plane diameters, while also indicating nodule depth and achieving a 21% reduction in missed nodules.1 The nodules are assesed based on Lung RADS 2022*.
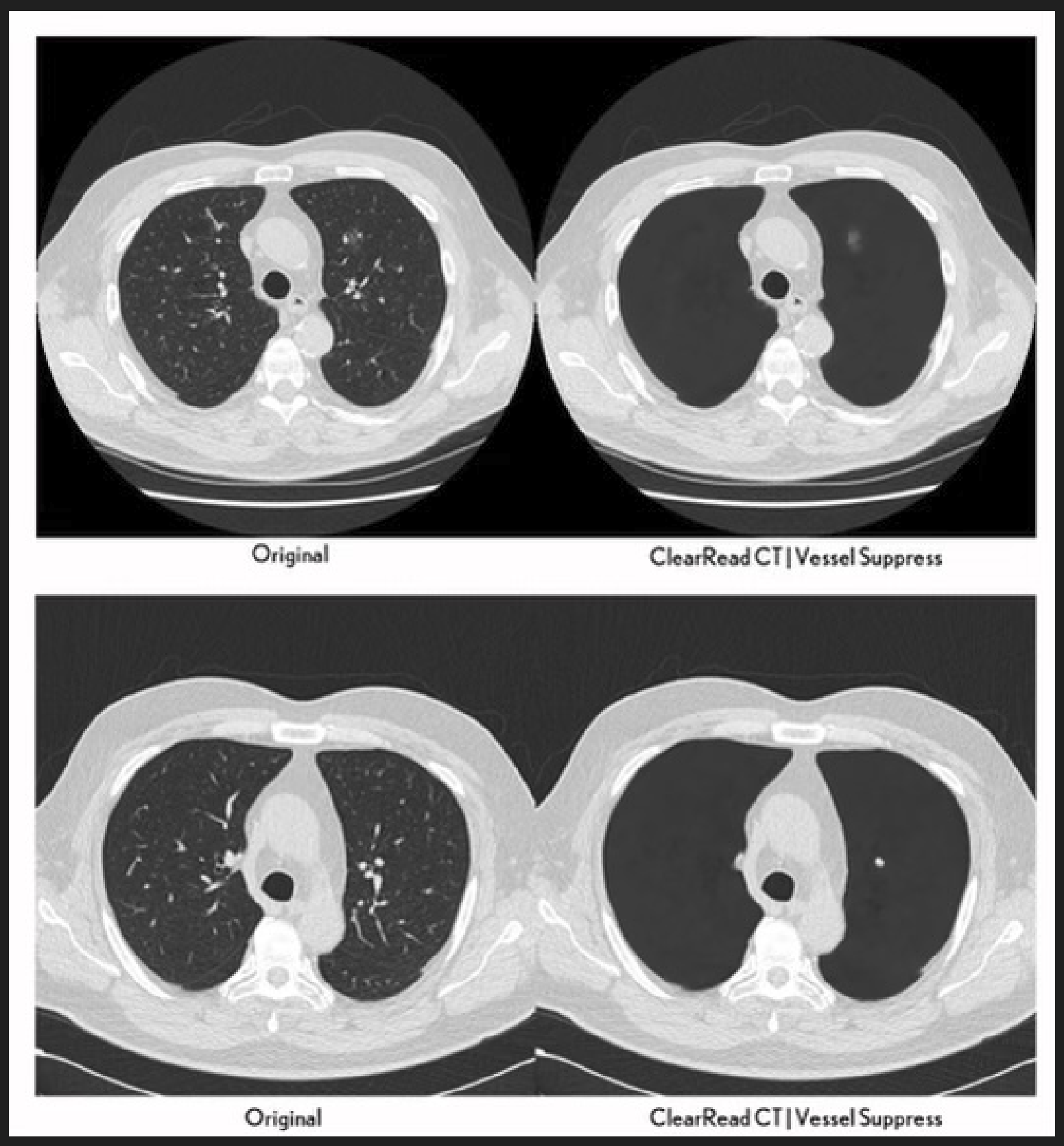
Utilizes unique suppression technology to enhance the interpretation of thoracic imaging, aiding radiologists in detecting cardio-thoracic diseases and improving nodule characterization.3
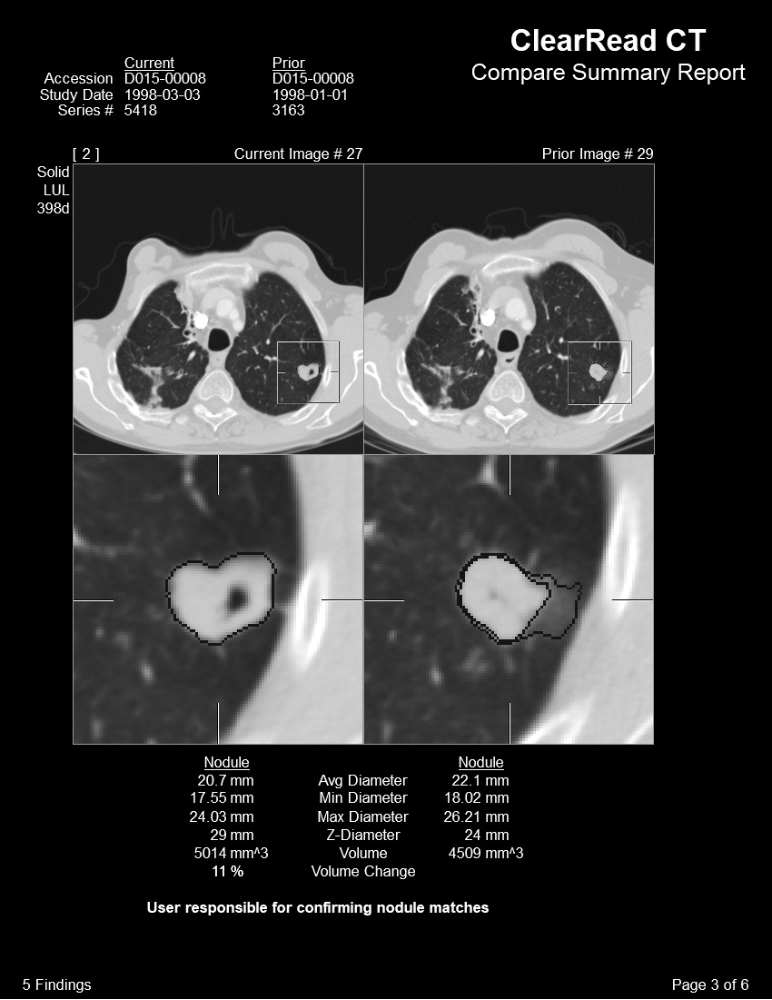
Using automated current and prior precision measurements, the system enhances efficiency, repeatability, and consistency across different readers, time of day, and workloads by automatically matching nodules identified in the current exam with those from a previous exam.
.bmp)
The CAC system employs deep learning AI technology to measure coronary artery calcification and determine the risk associated with coronary artery disease.
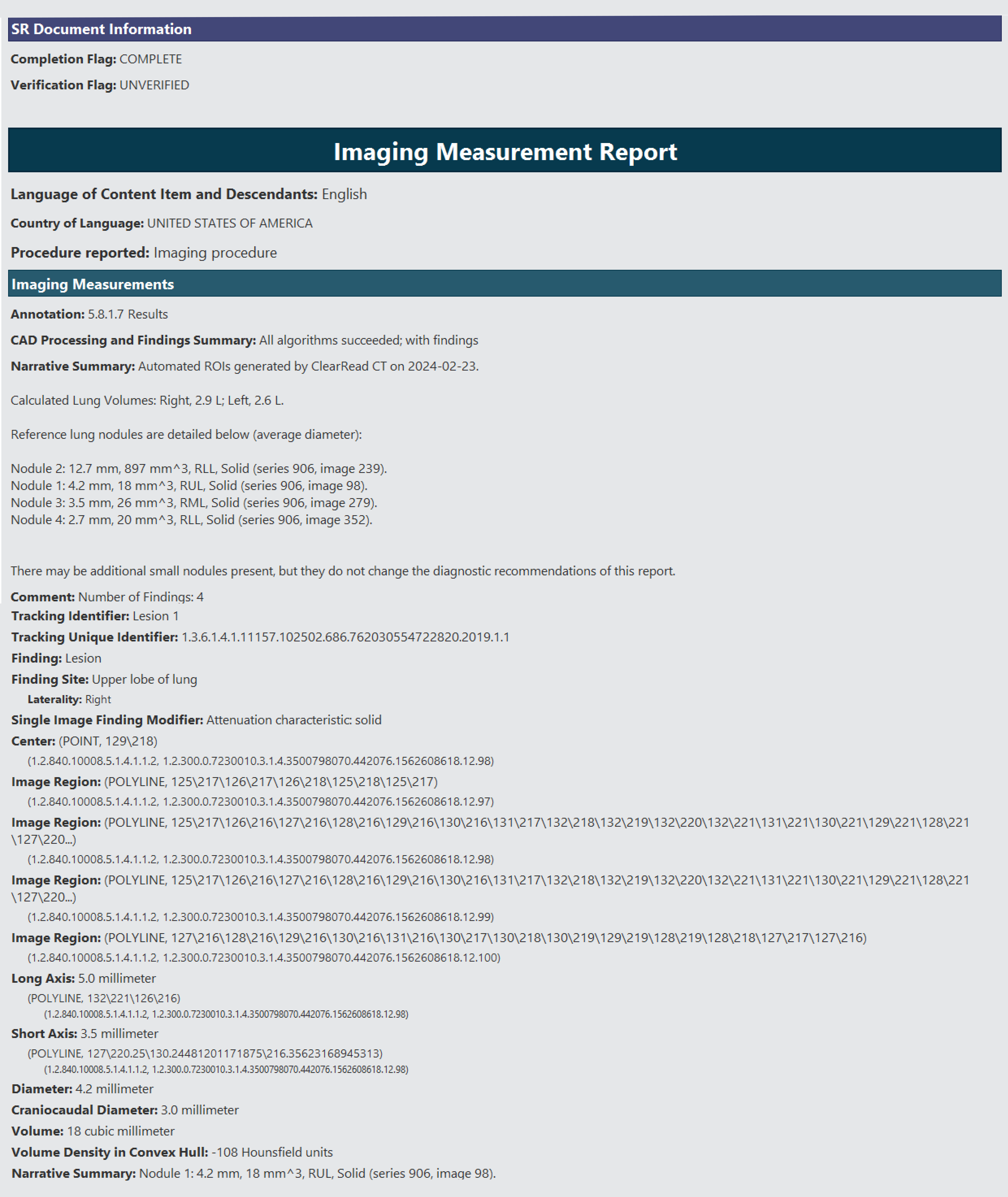
The ClearRead CT structured report supports a variety of formats, including DICOM for detailed imaging, PDF for easy report sharing, JPEG/PNG for individual image exports, and integration with PACS and web-based viewers, ensuring comprehensive accessibility and usability of CT scan results.
*pending CE mark
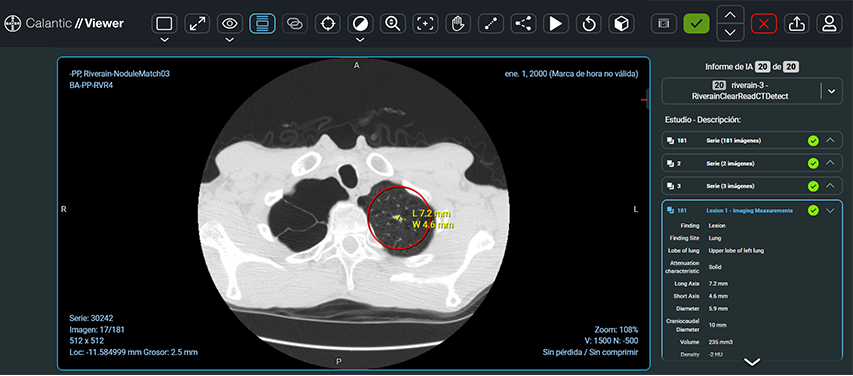
ACCEPT / REJECT WORKFLOW
Radiologists decide whether to accept or reject findings.
Findings can be selected from the Findings table or scrolled through using the left and right arrow buttons.
Once a radiologist is satisfied with the accepted and rejected findings, they can finalize the results, after which they cannot be amended.
AI platfrom from Bayer is ideal for radiology centers looking to modernize and optimize operations, offering several key benefits:
-

Unified Viewer
-

Cloud Deployment
Streamlines image visualization and integrates with existing systems.
Simplifies implementation and reduces IT maintenance.
-

Scalability
Adapts capacity to demand, allowing for cost-effective growth.
-

System Maintenance
Minimizes complexity by eliminating hardware management.
-

Access to Complementary Solutions
Through the Calantic™ Marketplace & MyApps, clinical users can evaluate, select, and deploy AI-enabled applications.
-

High System Availability
Ensures operational continuity with redundancy and disaster recovery, minimizing downtime risks that could affect patient care.
-

Reliable Technical Support
Backed by Bayer’s expertise in radiology, offering robust technical support and compliance assistance in cybersecurity and regulatory standards across EMEA.
-

Vendor Neutrality
Facilitates integration without altering existing infrastructure.
"Riverain’s ClearRead CT has been deployed as a part of our routine Chest CT exams, including patients in our Lung Cancer Screening Program. The ClearRead CT technology has helped us to detect lung nodules that may have otherwise been missed. Based upon our early experience, the workflow is faster and more accurate than existing technologies."
- Jared Christensen
- MD, MBA, Division Chief of Cardiothoracic Imaging and
- Director of Duke’s Lung Cancer Screening Program
References
1. Martini K, Blüthgen C, Eberhard M, et al. Impact of Vessel Suppressed-CT on Diagnostic Accuracy in Detection of Pulmonary Metastasis and Reading Time. Acad Radiol. 2021 Jul;28(7):988-994. doi: 10.1016/j.acra.2020.01.014. Epub 2020 Feb 6. PMID: 32037256.
2. Lo, S. B., Freedman, M. T., Gillis, L. B., White, C. S., & Mun, S. K. (2018). JOURNAL CLUB: Computer-Aided Detection of Lung Nodules on CT With a Computerized Pulmonary Vessel Suppressed Function. American Journal of Roentgenology, 210(3), 480–488. doi: 10.2214/ajr.17.18718.
3. Singh, Ramandeep, et. al. Effect of Artificial Intelligence Based Vessel Suppression and Automatic Detection of Part-Solid and Ground-Glass Nodules on Low-Dose Chest CT. RSNA 2018
© 2024 Bayer
The descriptions, claims and visuals for all non-Bayer owned applications originate and are obtained from the application providers, or excerpted from publicly available information. Bayer is providing this content for your information, but does not own and control such content.
BAYER, the Bayer Cross and Calantic are trademarks owned by Bayer and/or registered in the U.S. and/or other countries. Other trademarks and company names mentioned herein are properties of their respective owners.
This is intended to provide information for healthcare professionals and other relevant decision makers in the European Union, United Kingdom and Switzerland. Not all offerings are available in all markets. For availability specific to your country please contact your local Bayer representative. These materials are exclusively for professional use.
Calantic is ISO 27001 certified. The product Calantic Viewer has CE marking (CE2797). Complies with medical device legislation. Consult the operating instructions of a product for its intended use, characteristics and performance, contraindications, name of the manufacturer or its authorized representative and other essential information. The operating instructions of the product Calantic Viewer can be found on https://eifu.radiology.bayer.com/medicaldevice
You are encouraged to report adverse events; reporting information can be found on https://www.bayer.com/en/pharma/report-side-effect
Any individuals depicted in this website are actors and not actual health care providers or patients. All patient data is fictitious. Images are an artistic representation for illustrative purposes only.


